

REPATHA® (evolocumab) Product Profile
EASE OF USE with Repatha®
Offer your patients simple dosing* and convenient self-administration† in their own homes1
One click, that's it.1 Just one 140 mg injection SC every 2 weeks†1 No titration and no dose adjustment needed for most patients†1
One click, that's it.1 Just one 140 mg injection SC every 2 weeks†1
No titration and no dose adjustment needed for most patients†1
Repatha® is self-administered by the patient, meaning that once they are appropriately trained by a healthcare professional, there is no additional burden on you or your colleagues with respect to initiation and subsequent administration1
Amgen can support you with offering appropriate injection training.
Below you can find resources to assist you and your patients. The patient information and support booklet provides clarity on why your patient has been prescribed Repatha® and includes a step-by-step guide to self-administration. Our patient website has a clear self injection guidance video and some useful links. If you would like to discuss additional ways we can support you and your patients, let us know via the "Contact Amgen for Support" button.

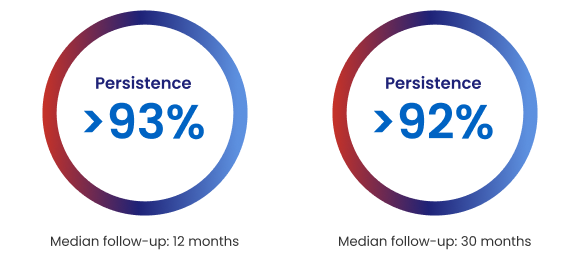
Persistence and compliance with Repatha®
Real-world data (HEYMANS study) from 1921 European adults at 12 months and 999 European adults at 30 months initiating Repatha® in routine clinical practice show:‡2
*For adult patients with primary hypercholesterolaemia or mixed dyslipidaemia or established atherosclerotic cardiovascular disease, the recommended dose is either 140 mg every two weeks or 420 mg once a month. For the 420 mg dose, three pre-filled syringes will be used. In adults and adolescents aged 12 years and over with HoFH, the initial recommended dose is 420 mg QM. After 12 weeks of treatment, dose frequency can be up-titrated to 420 mg Q2W if a clinically meaningful response is not achieved. Patients on apheresis may initiate treatment with 420 mg Q2W to correspond with their apheresis schedule. No dose adjustment is necessary in elderly patients, patients with renal impairment, or patients with mild hepatic impairment.1
†Repatha® is intended for patient self-administration after proper training. Administration of Repatha® can also be performed by an individual who has been trained to administer the product.1
‡HEYMANS was an observational serial chart review of Repatha® use in European subjects with hyperlipidaemia initiating Repatha® as part of routine clinical management and within local reimbursement criteria. Assessment of persistence and adherence was a secondary objective of the study. The HEYMANS study was initially designed for a 12-month follow-up of 1951 patients, but the protocol was amended in February 2018 to extend to up to 30 months with a subset of 1136 patients. The data is therefore provided in for two time periods: 0-12 months and 12-30 months.
Abbreviations
HoFH, Homozygous familial hypercholesterolaemia; LDL-C, low-density lipid cholesterol; QM, monthly; Q2W, every two weeks; SC, subcutaneous.
-
References
- Repatha® (evolocumab), Summary of Product Characteristics.
- Ray K et al. Atherosclerosis. 2023;366:14-21. doi:10.1016/j.atherosclerosis.2023.01.002
-
Adverse Event Reporting InformationAdverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Amgen Limited on +44 (0) 1223 436441.

