

REPATHA® (evolocumab) Study Data
FOURIER:1
The pre-specified secondary analysis shown here examined CV outcomes amongst 22,320 FOURIER patients with a prior MI, classified as either recent (≤12 months prior to randomisation; (n=5,711) or remote (>12 months; (n=16,609).2 In the recent MI group, treatment with Repatha® was initiated a median of 4.8 months after the index event.2

Primary Endpoint
15% relative risk reduction in major CV events (composite of CV death, MI, stroke, hospitalisation for unstable angina or coronary revascularisation) vs placebo p<0.001, HR 0.85 (95% CI 0.79-0.92). ARR 1.5%, 1,344 (9.8%) Repatha® + statin events vs 1,563 (11.3%) placebo + statin events1
-
CV RESULTS
REPATHA®
+ statin reduced LDL-C by 59% vs placebo + statin in high risk patients1*
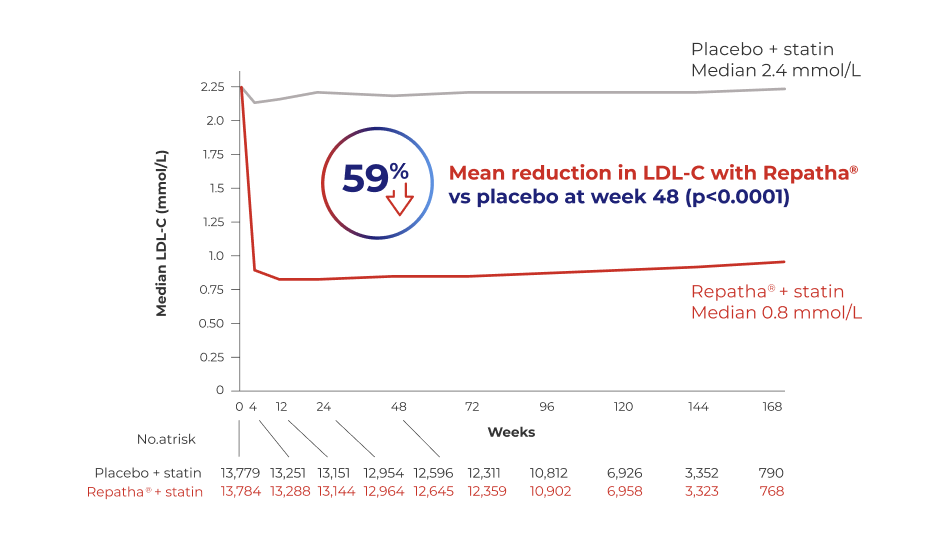
Adapted from Sabatine MS et al., N Eng J Med. 2017.
*Patients with ASCVD and LDL-C ≥ 1.8 mmol/L who were receiving statin therapy.
Repatha® patients could either be treated with Repatha® 140 mg Q2W or 420 mg QM
-
CV OUTCOMES
IMPROVED CV OUTCOMES
Repatha® + statin reduced the risk of CV death, MI or stroke by 20% vs. placebo + statin1
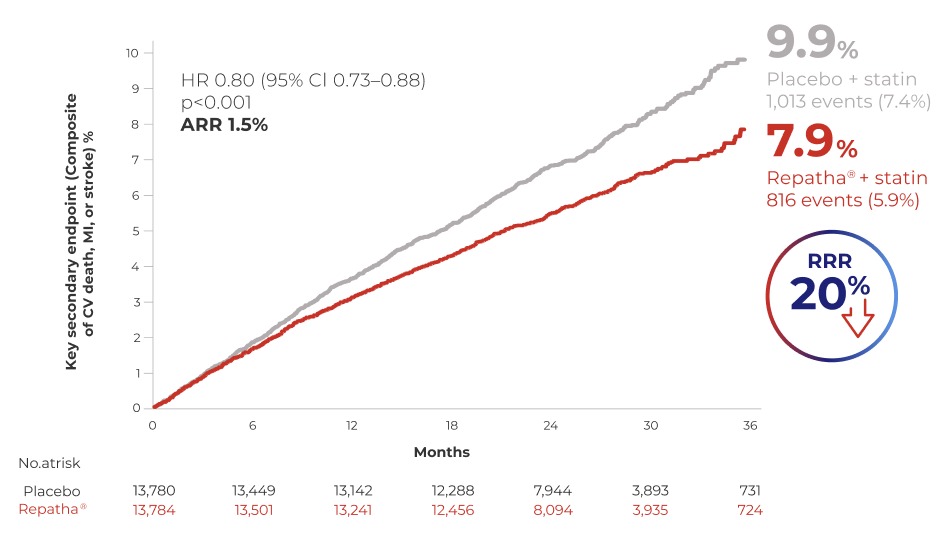
Repatha 140 mg Q2W or 420 mg QM, doses are clinically equivalent.
Adapted from Sabatine MS et al. N Eng J Med. 2017.
-
RECENT MI
Efficacy of Repatha® on CV outcomes in patients with recent MI*
Repatha® + statin reduced the risk of CV death, MI or stroke vs. placebo + statins in patients with recent MI*2
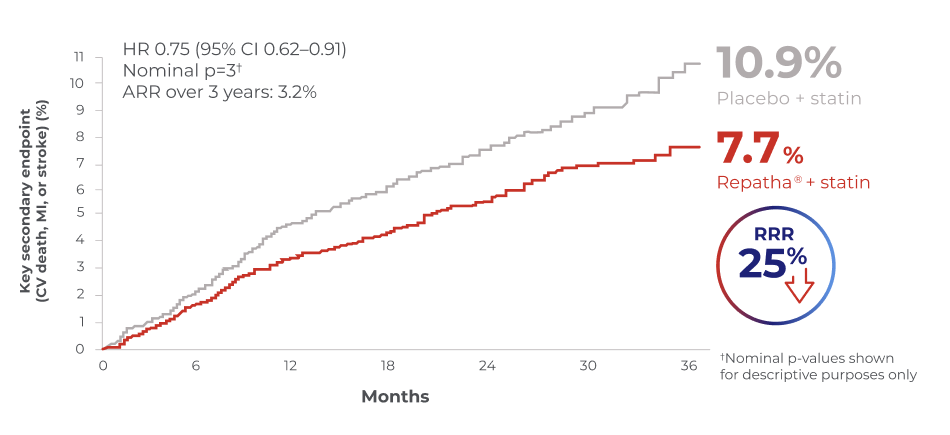
*≤12 months prior to randomisation; n=5,711
Adapted from Gencer, B et al. JAMA Cardiol. 2020.
-
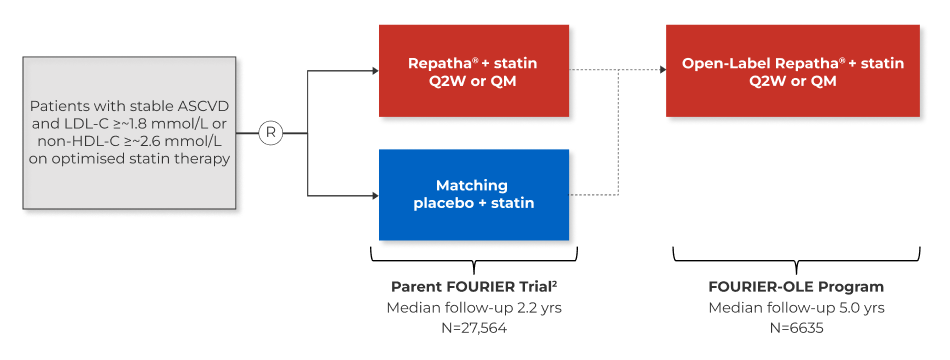
OPEN LABEL EXTENSION
Long term treatment with Repatha® in patients with established atherosclerotic cardiovascular disease:
Study design of the fourier-ole (open-label extension) studies1
-
LDL-C REDUCTION
Fourier-ole trial1
LDL-C Reduction
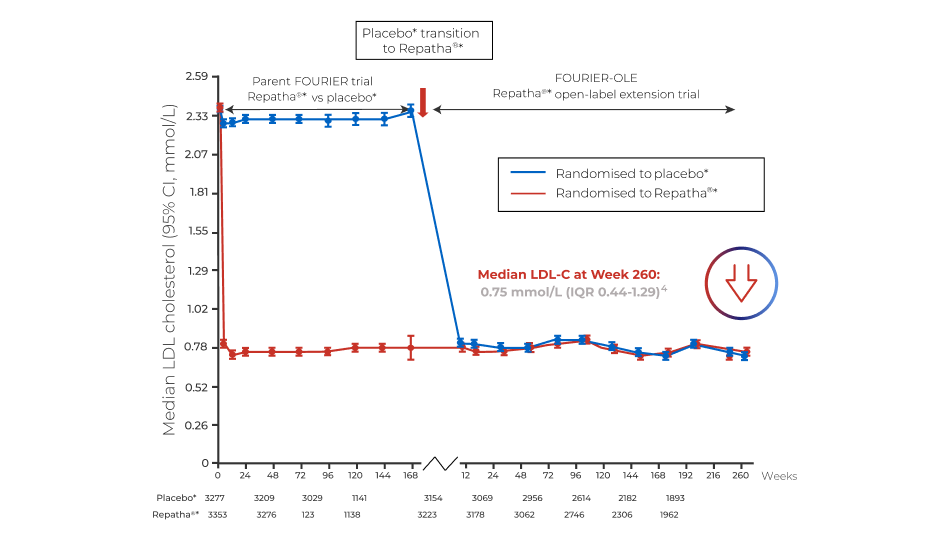
*in combination with a statin1.
Adapted from O'Donoghue ML et al., Circulation. 2022
-
SAFETY & TOLERABILITY
Fourier-ole trial1
Long-term safety and tolerability
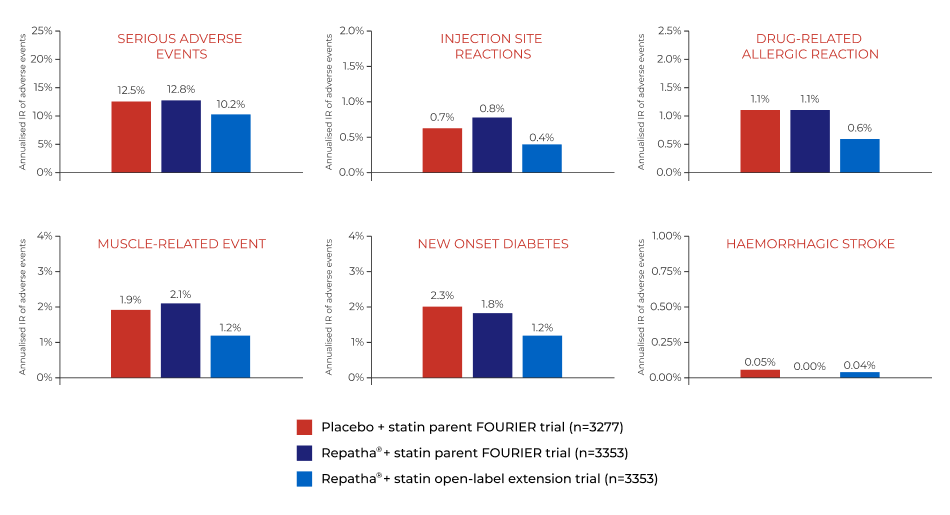
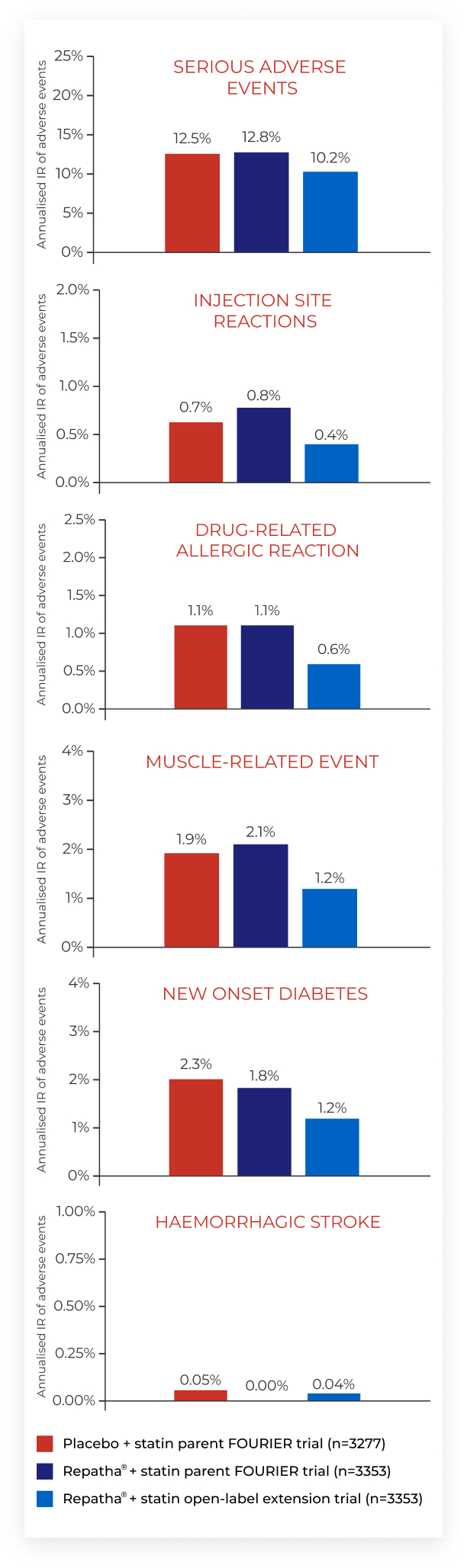
Abbreviations
ASCVD, atherosclerotic cardiovascular disease; ARR, absolute risk reduction; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; HR, hazard ratio; IR, incidence rate; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiovascular events; MI, myocardial infarction; NS, non-significant; OLE, open-label extension; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; QM, monthly; Q2W, every two weeks: R, randomised.
-
References
- Sabatine MS, et al., N Engl J Med. 2017;376:1713-1722.
- Gencer B, et al., JAMA Cardiol. 2020;5(8):952-957.
- O'Donoghue ML, et al., Circulation. 2022;146:1109-1119
- O'Donoghue ML, et al. Circulation. 2022;146:1109-1119 supplement
-
Adverse Event Reporting InformationAdverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Amgen Limited on +44 (0) 1223 436441.


